SIGNIFOR® LAR is indicated for the treatment of patients with acromegaly who have had an inadequate response to surgery and/or for whom surgery is not an option.1
SIGNIFOR LAR mechanism of action
SIGNIFOR LAR binds with high affinity to SST receptors 5 and 2, which are expressed on pituitary adenoma cells1
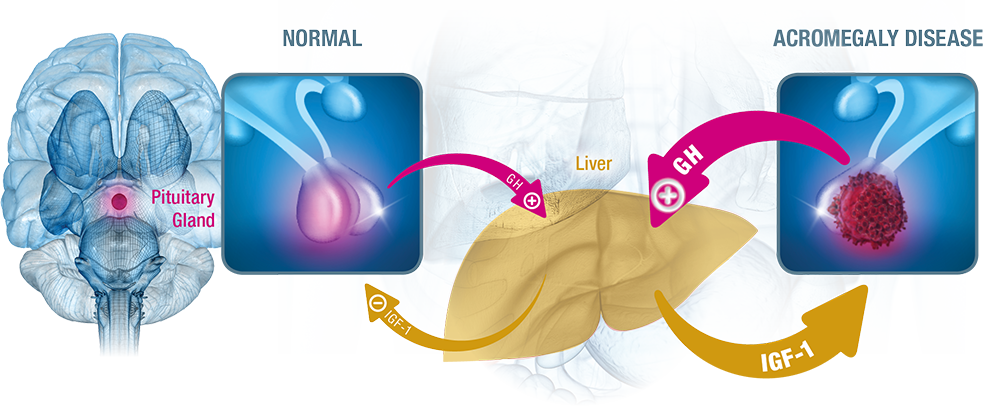
Signs and symptoms of acromegaly stem from an imbalance in the normal regulatory mechanism2-5:
- A pituitary adenoma secretes excessive amounts of GH over a prolonged period of time.
- Secretion of GH by the pituitary gland into the bloodstream stimulates the liver to overproduce IGF-1.
- High levels of IGF-1, in turn, signal the pituitary gland to reduce GH production.
- This disruption of hormonal balance can lead to signs and symptoms of acromegaly such as bone overgrowth and organ enlargement.
SIGNIFOR LAR binds with high affinity to SST receptors 5 and 2, which are expressed on pituitary adenoma cells1
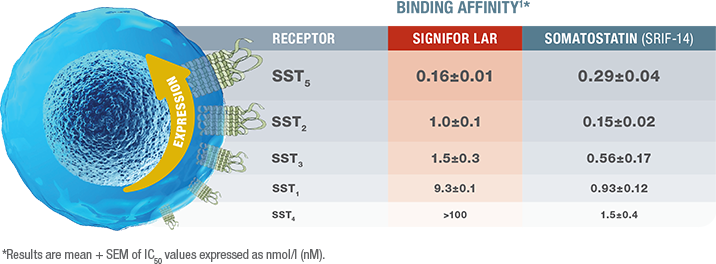
- SIGNIFOR LAR has the highest affinity for SST5 and SST2 and lower affinity for the other SST receptors1
- This effect has been shown to lower GH and IGF-1 levels in patients with acromegaly in vivo1
SST receptors are also expressed on pancreatic cells; their activation decreases insulin secretion6-10
- In vitro studies of human pancreatic cells have shown that when pancreatic SST receptors are activated insulin secretion is suppressed7,8
- In clinical studies of healthy volunteers, treatment with the SST analog SIGNIFOR LAR decreased total plasma insulin levels9,10
GH=growth hormone; IC50=concentration that inhibits 50%; IGF-1=insulin-like growth factor 1; SEM=standard error of mean; SST=somatostatin.
SIGNIFOR LAR efficacy in acromegaly
In previously untreated patients
SIGNIFOR LAR restore balance at the source
Results from clinical studies
Drug-naive patients treated with SIGNIFOR LAR were more likely to achieve biochemical normalization than those who received another SSA active comparator1
CLINICAL TRIAL: EFFICACY IN DRUG-NAIVE PATIENTS1,11
PRIMARY ENDPOINT
IN DRUG-NAIVE PATIENTS
Percentage of patients who achieved biochemical normalization1
(GH level <2.5 mcg/L and normalized IGF-1 at month 12)
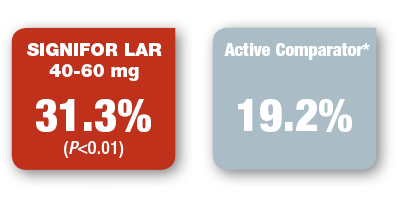
*The maximum dose approved for use in the US was not used in this trial, but the majority of patients were receiving the dose most commonly used in the US to treat acromegaly.
STUDY DESIGN: DRUG-NAIVE PATIENTS WITH ACROMEGALY1
A multicenter, randomized, double-blind study was conducted to assess the safety and efficacy of SIGNIFOR LAR in patients with active acromegaly. A total of 358 patients naive to drugs used to treat acromegaly were randomized in a 1:1 ratio to SIGNIFOR LAR or another SSA active comparator. Randomization was stratified based on previous pituitary surgical status (eg, at least 1 prior pituitary surgery versus no prior pituitary surgery).
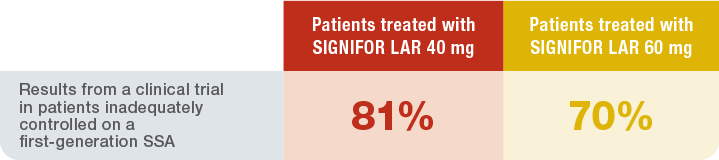
In patients who were inadequately controlled on a first-generation SSA11
Patients who were inadequately controlled on a first-generation SSA who switched to SIGNIFOR LAR experienced incremental benefits
Patients inadequately controlled on another SSA who switched to SIGNIFOR LAR treatment were more likely to experience biochemical control than those who remained on their previous SSA1
CLINICAL TRIAL: EFFICACY IN PATIENTS INADEQUATELY CONTROLLED†‡ AFTER 6 MONTHS TAKING A FIRST-GENERATION SSA1,11
PRIMARY ENDPOINT IN INADEQUATELY CONTROLLED PATIENTS
Percentage of patients who achieved biochemical normalization1
(GH level <2.5 mcg/L and normalized IGF-1 at month 6§)

†For one active comparator, the maximum approved US dose was not used, but most patients received the most common US dose for acromegaly.
‡Inadequate control defined as GH concentration of >2.5 mcg/L (ie, mean of 5 samples over 2 hours) and sex- and age-adjusted IGF-1 level >1.3 times upper limit of normal (ULN).
§Primary endpoint (patients with IGF-1 < lower limit of normal [LLN] were not considered as "responders").
STUDY DESIGN: PATIENTS WITH ACROMEGALY INADEQUATELY CONTROLLED ON A FIRST-GENERATION SSA1,11
A multicenter, randomized, 3-arm study was conducted in patients with acromegaly inadequately controlled on SSAs. Patients were randomized to double-blind SIGNIFOR LAR 40 mg (n=65) or SIGNIFOR LAR 60 mg (n=65) or to continued open-label pretrial SSA therapies at maximal or near maximal doses (n=68). A total of 181 patients completed the 6-month trial.
Percentage of patients with acromegaly that was inadequately controlled with a first generation SSA in whom tumor volume decreased or did not change1*
- The median change from baseline in tumor volume was -10.4% (range: -74.5% to 19.4%) for SIGNIFOR LAR 40 mg and -6.3% (range: -66.7% to 14.5%) for SIGNIFOR LAR 60 mg
*Tumor volume changes from baseline as assessed by MRI at month 6.
MRI=magnetic resonance imaging.
Percentage of drug naive patients with acromegaly in whom tumor volume decreased or did not change1†
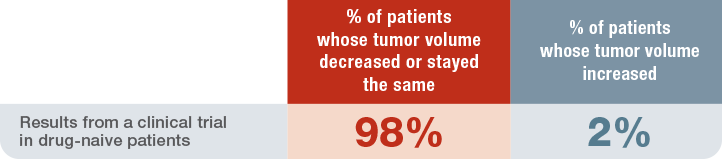
- The median change in tumor volume with SIGNIFOR LAR was -40% (range: -97.6% to 16.9%)
†Tumor volume changes from baseline as assessed by MRI at month 12.
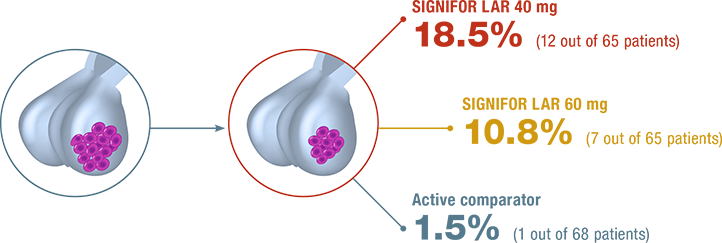
Impact on tumor volume in patients previously inadequately controlled on other first-generation SSAs11,12
PERCENTAGES OF PATIENTS WHO ACHIEVED TUMOR REDUCTION OF ≥25%12
Adverse reactions in acromegaly
Documented in a clinical trial in drug-naive patients with acromegaly1
Adverse reactions occurring in patients exposed to SIGNIFOR LAR who were previously naive to drug therapy (n=178)

*Diabetes mellitus includes the terms diabetes mellitus and type 2 diabetes mellitus.
SEE SECTION 6.1 IN FULL PRESCRIBING INFORMATION FOR COMPLETE ADVERSE REACTION TABLE.
Documented in a clinical trial in patients with acromegaly who were previously inadequately controlled on other SSAs1
Adverse reactions occurring in patients exposed to SIGNIFOR LAR who were previously treated with a first-generation SSA11 (n=125)
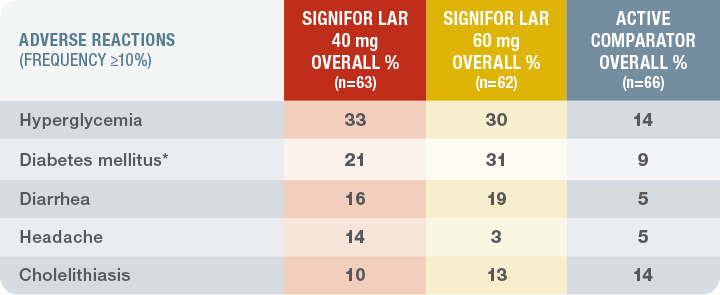
*Diabetes mellitus includes the terms diabetes mellitus and type 2 diabetes mellitus.
SEE SECTION 6.1 IN FULL PRESCRIBING INFORMATION FOR COMPLETE ADVERSE REACTION TABLE.
Dosing and administration for acromegaly
SIGNIFOR LAR is an intramuscular injection in the left or right gluteal muscle, and is administered by a physician or nurse, providing the opportunity to encourage ongoing adherence and to support patients throughout the course of treatment.1
1 INJECTION EVERY 4 WEEKS (28 DAYS)1
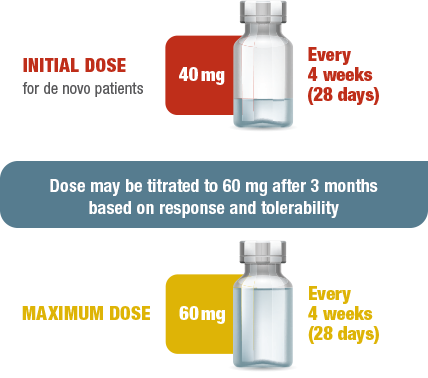
Intramuscular SIGNIFOR LAR dosing and titration1
- The long-acting formulation of intramuscular SIGNIFOR LAR provides 4 weeks (28 days) of medication release with a single injection1
- The clinical benefits of SIGNIFOR LAR should be evaluated after the first 3 months of treatment and periodically thereafter1
- Management of suspected adverse reactions or over-response to treatment (age- and sex-adjusted IGF-1 less than the lower limit of normal) may require dose reduction, interruption, or discontinuation of treatment with SIGNIFOR LAR1
References: 1. SIGNIFOR LAR (pasireotide) for injectable suspension, for intramuscular use [prescribing information]. Lebanon, NJ: Recordati Rare Diseases Inc.; 2020. 2. Christofides EA. Clinical importance of achieving biochemical control with medical therapy in adult patients with acromegaly. Patient Prefer Adherence. 2016;10:1217-1225. 3. Carmichael JD, Bonert VS, Nuño M, Ly D, Melmed S. Acromegaly clinical trial methodology impact on reported biochemical efficacy rates of somatostatin receptor ligand treatments: a meta-analysis. J Clin Endocrinol Metab. 2014;99(5):1825-1833. 4. Carroll PV, Jenkins PJ. Acromegaly. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc.; 2016. 5. National Institutes of Health. US National Library of Medicine. Acromegaly.https://www.niddk.nih.gov/-/media/Files/Endocrine-Diseases/Acromegaly_508.pdf?la=en&hash=496113F1BCD2A416D4FED99108A2E854. Updated May 2008. Accessed February 13, 2020. 6. Silverstein JM. Hyperglycemia induced by pasireotide in patients with Cushing’s disease or acromegaly. Pituitary. 2016;19(5):536-543. 7. Zambre Y, Ling Z, Chen MC, et al. Inhibition of human pancreatic islet insulin release by receptor-selective somatostatin analogs directed to somatostatin receptor subtype 5. Biochem Pharmacol. 1999;57(10):1159-1164. 8. Singh V, Brendel MD, Zacharias S, et al. Characterization of somatostatin receptor subtype-specific regulation of insulin and glucagon secretion: an in vitro study on isolated human pancreatic islets. J Clin Endocrinol Metab. 2007;92(2):673-680. 9. Breitschaft A, Hu K, Hermosillo Reséndiz K, Darstein C, Golor G. Management of hyperglycemia associated with pasireotide (SOM230): healthy volunteer study. Diabetes Res Clin Pract. 2014;103(3):458-465. 10. Henry RR, Ciaraldi TP, Armstrong D, Burke P, Ligueros-Saylan M, Mudaliar S. Hyperglycemia associated with pasireotide: results from a mechanistic study in healthy volunteers. J Clin Endocrinol Metab. 2013;98(8):3446-3453. 11. Melmed S, Bronstein MD, Chanson P, et al. A consensus statement on acromegaly therapeutic outcomes. Nat Rev Endocrinol. 2018;14(9):552-561. 12. Gadelha MR, Bronstein MD, Brue T, et al. Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(11):875-884.


