Indications and Usage
SIGNIFOR LAR (pasireotide) is a somatostatin analog indicated for the treatment of:
- Patients with acromegaly who have had an inadequate response to surgery and/or for whom surgery is not an option.
- Patients with Cushing’s disease for whom pituitary surgery is not an option or has not been curative.
Important Safety Information
Warnings and Precautions
- Hyperglycemia, Diabetes, and Ketoacidosis: SIGNIFOR LAR can cause increases in blood glucose levels which are sometimes severe. Monitor glucose levels as clinically appropriate during therapy. There have been postmarketing cases of ketoacidosis with SIGNIFOR LAR in patients with history of diabetes and in patients without history of diabetes. Patients who develop significant hyperglycemia on SIGNIFOR LAR may require initiation of anti-diabetic treatment or adjustment in their current anti-diabetic treatment. The optimal treatment for the management of SIGNIFOR LAR-induced hyperglycemia is not known. If hyperglycemia cannot be controlled despite medical management, reduce the dose or discontinue SIGNIFOR LAR. If ketoacidosis is suspected, discontinue SIGNIFOR LAR and promptly evaluate and treat the patient. Please see sections 2.1, 5.1 in full Prescribing Information.
- Bradycardia and QT Prolongation: Bradycardia has been reported with the use of SIGNIFOR LAR (pasireotide). Patients with cardiac disease and/or risk factors for bradycardia, such as history of clinically significant bradycardia, high grade heart block, or concomitant use of drugs associated with bradycardia, should be monitored. Adjustments in the dose of drugs known to slow the heart rate (e.g., beta-blockers, calcium channel blockers) and correction of electrolyte disturbances may be necessary when initiating or during the course of SIGNIFOR LAR (pasireotide) treatment. Use with caution in at-risk patients; evaluate ECG and electrolytes prior to dosing and periodically while on treatment.
- Liver Test Elevations: Evaluate liver enzyme tests prior to and during treatment. Please see sections 2.1, 5.3 in full Prescribing Information.
- Cholelithiasis and Complications of Cholelithiasis: Monitor periodically. Discontinue if complications of cholelithiasis are suspected. Please see section 5.4 in full Prescribing Information.
- Pituitary Hormone Deficiency(ies): Monitor for occurrence periodically and treat if clinically indicated. Please see section 5.5 in full Prescribing Information.
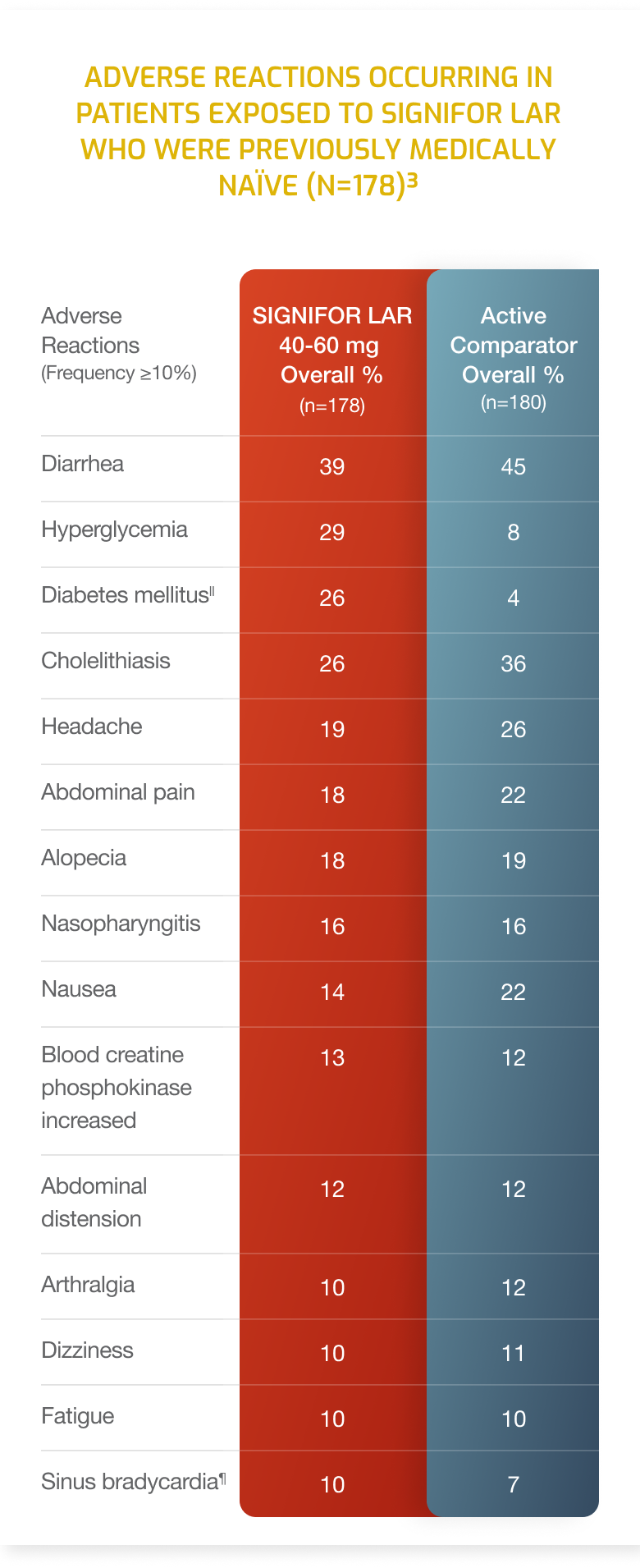
Adverse Reactions
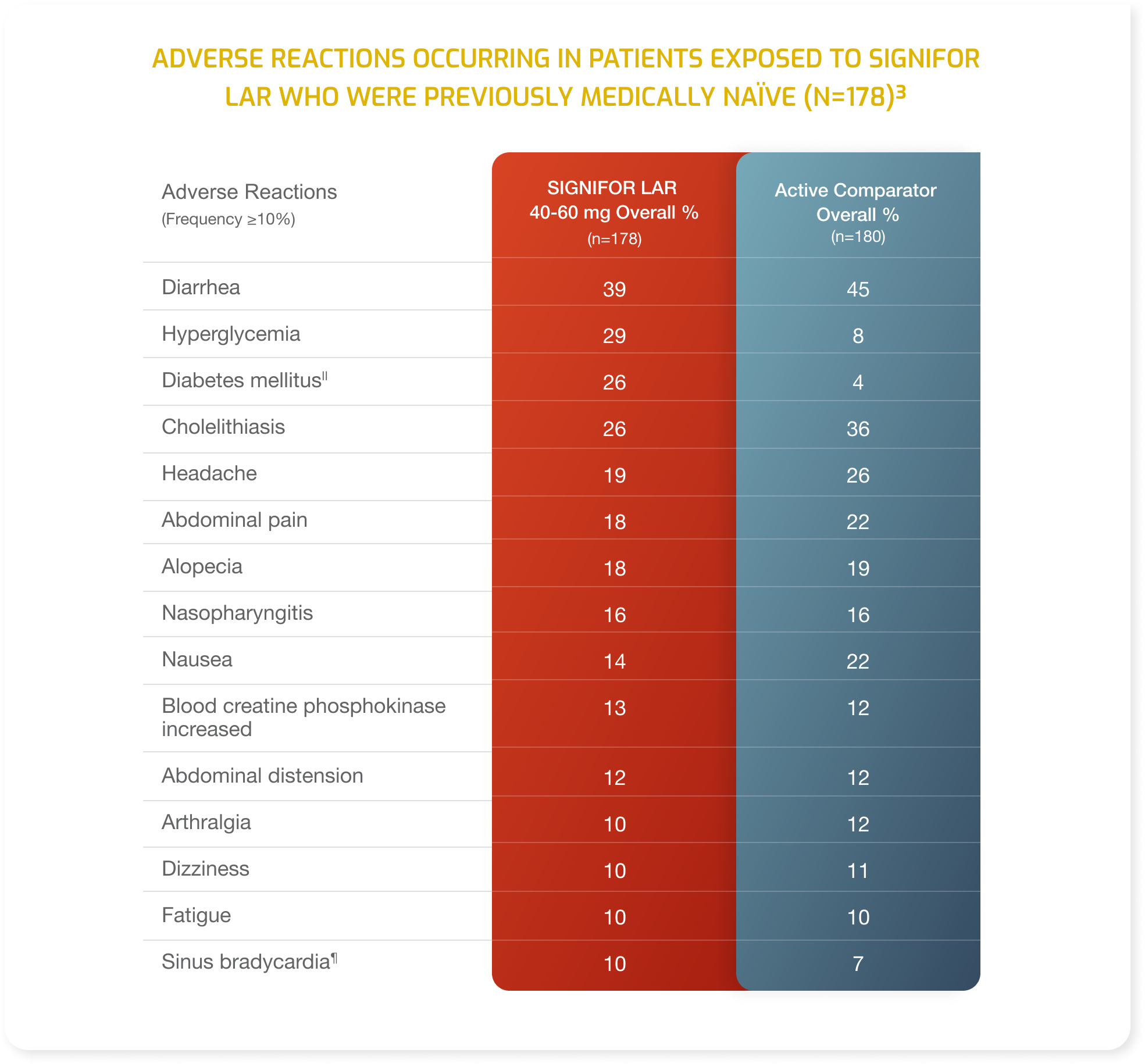
- Adverse drug reactions associated with SIGNIFOR LAR (pasireotide) and occurring in ≥20% of patients were diarrhea, cholelithiasis, hyperglycemia and diabetes mellitus.
- To report SUSPECTED ADVERSE REACTIONS, contact Recordati Rare Diseases Inc. at 1-888-575- 8344, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Drugs that Prolong QT: Use with caution in patients who are at significant risk of developing QTc prolongation.
- Cyclosporine: Consider additional monitoring.
- Bromocriptine: Consider bromocriptine dose reduction.
Females and Males of Reproductive Potential
- Advise premenopausal females of the potential for an unintended pregnancy.
References: 1. Gatto F, Barbieri F, Arvigo M, et al. Biological and biochemical basis of the differential efficacy of first and second generation somatostatin receptor ligands in neuroendocrine neoplasms.
Int J Mol Sci. 2019;20(16):3940.
2. Poullot A-G, Chevalier N. New options in the treatment of Cushing’s disease: a focus on pasireotide.
Res Rep Endocr Disord. 2013;3:31-38.
3. SIGNIFOR LAR (pasireotide) for injectable suspension, for intramuscular use [prescribing information]. Lebanon, NJ: Recordati Rare Diseases Inc.; 2020.
4. Acromegaly. UCLA Health System. https://www.uclahealth.org/medical-services/surgery/endocrine-surgery/patient-resources/patient-education/endocrine-surgery-encyclopedia/acromegaly. Accessed August 23, 2022.
5. Broder MS, Chang E, Cherepanov D, Neary MP, Ludlam WH. Incidence and Prevalence of Acromegaly in the United States: A Claims-Based Analysis. AACE Endocrine Practice. https://www.endocrinepractice.org/article/S1530-891X(20)35591-9/pdf. Published November 1, 2016. Accessed August 23, 2022.
6. Christofides EA. Clinical importance of achieving biochemical control with medical therapy in adult patients with acromegaly.
Patient Prefer Adherence. 2016;10:1217-1225.
7. Acromegaly. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/endocrine-diseases/acromegaly. Accessed August 23, 2022.
8. Carmichael JD, Bonert VS, Nu.o M, Ly D, Melmed S. Acromegaly clinical trial methodology impact on reported biochemical efficacy rates of somatostatin receptor ligand treatments: a meta-analysis.
J Clin Endocrinol Metab. 2014;99(5):1825-1833.
9. Carroll PV, Jenkins PJ. Acromegaly. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc.; 2016
10. Giustina A, Barkhoudarian G, Beckers A, et al. Multidisciplinary management of acromegaly: A consensus.
Rev Endocr Metab Disord. 2020;21(4):667-678.
11. Katznelson L, Laws ER Jr, Melmed S, et al. Endocrine Society Acromegaly: an Endocrine Society clinical practice guideline.
J Clin Endocrinol Metab. 2014;99(11):3933-3951.
12. Lavrentaki A, Paluzzi A, Wass JA, Karavitaki N. Epidemiology of acromegaly: review of population studies.
Pituitary. 2017;20(1):4-9.
13. SOMATULINE® DEPOT (lanreotide) injection, for subcutaneous use [prescribing information]. Cambridge, MA: Ipsen Biopharmaceuticals, Inc.; 2019
14. SANDOSTATIN LAR DEPOT (octreotide acetate) for injectable suspension, for gluteal intramuscular use [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2021.
15. Coopmans EC, Muhammad A, van der Lely AD, et al. How to position pasireotide LAR treatment in acromegaly.
J Clin Endocrinol Metab. 2019;104(6):1978-1988.
16. Shanik MH, Cao PD, Ludlam WH. Historical response rates of somatostatin analogues in the treatment of acromegaly: a systematic review. Endocr Pract. 2016;22(3):350-356.
17. Casar-Borota O, Heck A, Schulz S, et al. Expression of SSTR2a, but not of SSTRs 1, 3, or 5 in somatotroph adenomas assessed by monoclonal antibodies was reduced by octreotide and correlated with the acute and long-term effects of octreotide.
J Clin Endocrinol Metab. 2013;98(11):E1730-E1739.
18. Silverstein JM. Hyperglycemia induced by pasireotide in patients with Cushing’s disease or acromegaly.
Pituitary. 2016;19:536-543.
19. Zambre Y, Ling Z, Chen MC, et al. Inhibition of human pancreatic islet insulin release by receptor-selective somatostatin analogs directed to somatostatin receptor subtype 5.
Biochem Pharmacol. 1999;57(10):1159-1164.
20. Singh V, Brendel MD, Zacharias S, et al. Characterization of somatostatin receptor subtype-specific regulation of insulin and glucagon secretion: an in vitro study on isolated human pancreatic islets.
J Clin Endocrinol Metab. 2007;92(2):673-680.
21. Breitschaft A, Hu K, Hermosillo Res.ndiz K, Darstein C, Golor G. Management of hyperglycemia associated with pasireotide (SOM230): healthy volunteer study.
Diabetes Res Clin Pract. 2014;103(3):458-465.
22. Henry RR, Ciaraldi TP, Armstrong D, Burke P, Ligueros-Saylan M, Mudaliar S. Hyperglycemia associated with pasireotide: results from a mechanistic study in healthy volunteers.
J Clin Endocrinol Metab. 2013;98(8):3446-3453.
23. Gadelha MR, Bronstein MD, Brue T, et al. Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial.
Lancet Diabetes Endocrinol. 2014;2(11):875-884.
24. Colao A, Bronstein MD, Freda P, et al. Pasireotide versus octreotide in acromegaly: a head-to-head superiority study.
J Clin Endocrinol Metab. 2014;99(3):791-799.
25. Gadelha MR, Gu F, Bronstein MD, et al. Risk factors and management of pasireotide-associated hyperglycemia in acromegaly.
Endocr Connect. 2020;9(12):1178-1190.
26. American Diabetes Association. Standards of Medical Care in Diabetes-2020 Abridged for Primary Care Providers.
Clin Diabetes. 2020;38(1):10-38. doi:10.2337/cd20-as01.
27. Samson SL, Gu F, Feldt-Rasmussen U, Zhang S, Yu Y, et al. Managing pasireotide-associated hyperglycemia: a randomized, open-label, Phase IV study.
Pituitary. 2021;24(6):887-903.