SIGNIFOR® LAR is the first and only long-acting, pituitary-targeted therapy for Cushing’s disease with proven clinical efficacy.1,2
SIGNIFOR LAR mechanism of action
Target the receptors most responsible for ACTH oversecretion to help settle the storm of cortisol oversecretion3
In Cushing's disease, the HPA feedback loop is disrupted, leaving corticotropin-releasing hormone (CRH) and ACTH production uncontrolled. Pituitary adenomas are resistant to cortisol inhibition and oversecrete ACTH, stimulating the adrenal glands to produce excess cortisol.4
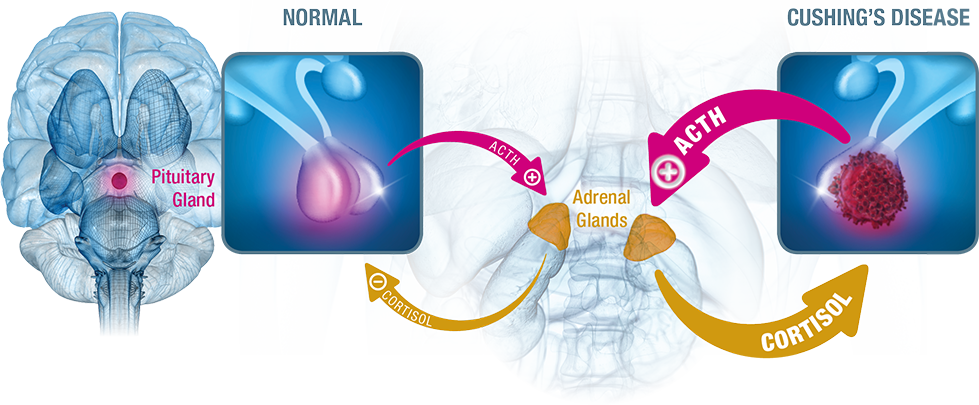
SIGNIFOR LAR predominantly targets SST receptors 5 and 23

SIGNIFOR LAR decreases cortisol levels through inhibition of ACTH secretion by targeting key somatostatin (SST) receptors of the pituitary adenoma cells3
- SIGNIFOR LAR has a pharmacologic profile that targets 4 of the 5 SST receptors, and has the highest binding affinity for SST5 and SST23
- SST5 is known to be the most overexpressed SST receptor in Cushing’s disease3
SST receptors are also expressed on pancreatic cells; their activation decreases insulin secretion7-11
- In vitro studies of human pancreatic cells have shown that when pancreatic SST receptors are activated insulin secretion is suppressed8,9
- In clinical studies of healthy volunteers, treatment with the SST analog SIGNIFOR LAR decreased total plasma insulin levels10,11
Because SIGNIFOR LAR predominantly targets SST receptors, and those receptors are overexpressed on pancreatic cells, hyperglycemia can occur and should be addressed3,7-11
- In clinical studies of Cushing’s disease, hyperglycemia occurred in 47% of patients and type 2 diabetes was observed in 27%3
- Blood glucose monitoring should be done weekly for the first 3 months after initiating SIGNIFOR LAR and weekly for the first 4 to 6 weeks after dose increases. Periodic monitoring should continue thereafter, as clinically appropriate3
- Most incidences of increased glucose levels in clinical trials occurred within the first 3 months of SIGNIFOR LAR treatment initiation3
- Patients who develop significant hyperglycemia may require initiation of antidiabetic therapy or adjustment in their current antidiabetic therapy per standard of care3
- If hyperglycemia cannot be controlled despite medical management, the dose of SIGNIFOR LAR should be reduced or treatment with SIGNIFOR LAR should be discontinued3
SIGNIFOR LAR efficacy in Cushing’s disease
SIGNIFOR LAR normalized cortisol levels for many patients3,12,13
- Treatment with SIGNIFOR LAR resulted in many patients seeing a decrease in urinary free cortisol (UFC), the diagnostic standard for Cushing’s disease3,12,13
Primary Endpoint
~40% OF PATIENTS ACHIEVED NORMALIZED UFC (mUFC ≤ULN)3,13
Proportions of patients (ITT) with mUFC concentrations ≤ULN at month 7
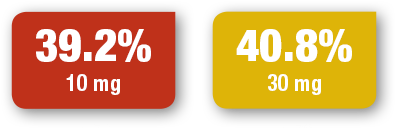
Study design: Efficacy and safety of SIGNIFOR LAR were assessed in a phase 3, randomized, double-blind, multicenter, 12-month study that compared two dose regimens of SIGNIFOR LAR in 150 patients with persistent or recurrent Cushing’s disease, or de novo patients who were not candidates for pituitary surgery.
At 4 months, patients whose mUFC had normalized (≤1.5 x ULN) continued on the randomized dose and patients with mUFC >1.5 x ULN underwent blinded dose increases (from 10 mg to 30 mg, or from 30 mg to 40 mg), unless tolerability concerns were present. Additional one-level dose increases were allowed at months 7 and 9 if mUFC was >1 x ULN.
SUPPORTIVE EFFICACY ANALYSIS3,14*
Response rate at month 7
(controlled + partially controlled)
*Percentages of patients with mUFC ≤ULN or ≥50% reduction from baseline. This is a less stringent endpoint than the primary endpoint.

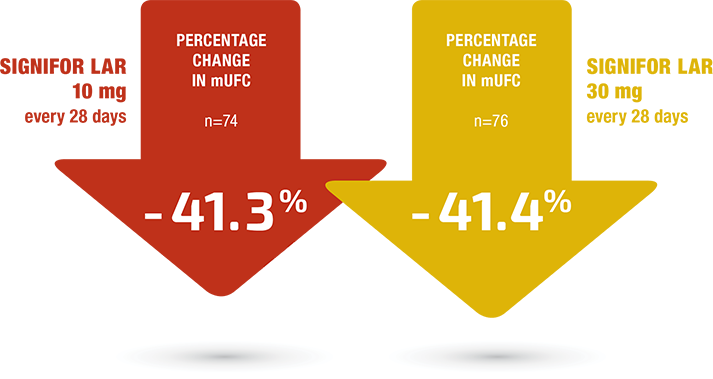
SIGNIFOR LAR reduced mUFC levels in both treatment groups3
SECONDARY ENDPOINT
Percentage change (median) from baseline in mUFC concentrations at month 73†
ITT=intent-to-treat population; mUFC=mean UFC; ULN=upper limit of normal.
†The baseline mUFC levels for the 10-mg arm and the 30-mg arm were 462.6 nmol/24 h and 477.1 nmol/24 h, respectively.
Hyperglycemia can occur and should be addressed3
Somatostatin (SST) receptors are also expressed on pancreatic cells; their activation decreases insulin secretion7-11
SIGNIFOR LAR CAN CAUSE INCREASES IN BLOOD GLUCOSE LEVELS, WHICH ARE SOMETIMES SEVERE. PATIENTS WITH POOR BASELINE GLYCEMIC CONTROL ARE AT HIGHER RISK OF DEVELOPING SEVERE HYPERGLYCEMIA3
- In clinical studies of Cushing’s disease, hyperglycemia occurred in 47% of patients and type 2 diabetes was observed in 27%3
- Blood glucose monitoring should be done weekly for the first 3 months after initiating SIGNIFOR LAR and weekly for the first 4 to 6 weeks after dose increases. Periodic monitoring should continue thereafter, as clinically appropriate3
- Most incidences of increased glucose levels in clinical trials occurred within the first 3 months of SIGNIFOR LAR treatment initiation3
- Patients who develop significant hyperglycemia may require initiation of antidiabetic therapy or adjustment in their current antidiabetic therapy per standard of care3
- If hyperglycemia cannot be controlled despite medical management, the dose of SIGNIFOR LAR should be reduced or treatment with SIGNIFOR LAR should be discontinued3

SEE SECTION 6.1 IN FULL PRESCRIBING INFORMATION FOR COMPLETE ADVERSE REACTION TABLE.
Adverse reactions in Cushing’s disease clinical trials
Safety profile documented in a randomized phase 3 clinical study among 150 patients with Cushing’s disease3
SIGNIFOR LAR was studied in a phase 3, randomized, double-blind, multicenter study to evaluate the safety and efficacy of 2 dose regimens of SIGNIFOR LAR over a 12-month treatment period in patients with persistent or recurrent Cushing’s disease, or de novo patients who were not considered candidates for pituitary surgery.
SIGNIFOR LAR safety profile
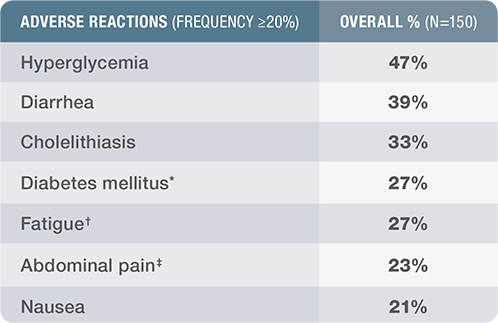
*Diabetes mellitus consists of the 2 terms: diabetes mellitus and type 2 diabetes mellitus.
†Fatigue includes the term asthenia.
‡Abdominal pain includes the term abdominal pain upper.
SEE SECTION 6.1 IN FULL PRESCRIBING INFORMATION FOR COMPLETE ADVERSE REACTION TABLE.
- Adverse drug reactions associated with SIGNIFOR LAR and occurring in ≥20% of patients were diarrhea, cholelithiasis, hyperglycemia, and diabetes mellitus
Dosing and administration
1 INJECTION EVERY 4 WEEKS (28 DAYS)3
Intramuscular SIGNIFOR LAR is administered into a gluteal muscle by a trained healthcare professional such as a physician or nurse.
- Each injection provides an opportunity to encourage adherence and provide support for patients throughout the course of treatment
- Schedule your patient’s next injection for 4 weeks (28 days) from the current injection date
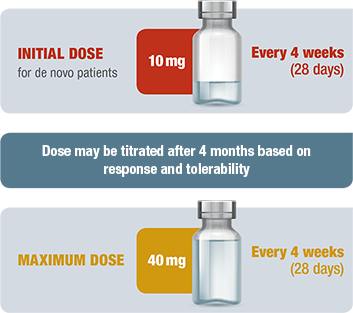
Intramuscular SIGNIFOR LAR dosing and titration3
- The long-acting formulation of intramuscular SIGNIFOR LAR provides 4 weeks (28 days) of medication release with a single injection
- The clinical benefits of SIGNIFOR LAR should be evaluated after the first 4 months of treatment and periodically thereafter
- Management of suspected adverse reactions or over-response to treatment (eg, cortisol levels below the lower limit of normal) may require dose reduction, interruption, or discontinuation of treatment with SIGNIFOR LAR
References: 1. Ciato D, Mumbach AG, Paez-Pereda M, Stalla GK. Currently used and investigational drugs for Cushing’s disease. Expert Opin Investig Drugs. 2017;26(1):75-84. 2. Langlois F, Chu J, Fleseriu M. Pituitary-directed therapies for Cushing’s disease. Front Endocrinol. 2018;9(164)1-12. 3. SIGNIFOR LAR (pasireotide) for injectable suspension, for intramuscular use [prescribing information]. Lebanon, NJ: Recordati Rare Diseases Inc.; 2020. 4. Lonser RR, Nieman L, Oldfield EH. Cushing’s disease: pathobiology, diagnosis, and management. J Neurosurg. 2017;126(2):404-417. 5. Neto LV, Machado Ede O, Luque RM, et al. Expression analysis of dopamine receptor subtypes in normal human pituitaries, nonfunctioning pituitary adenomas and somatotropinomas, and the association between dopamine and somatostatin receptors with clinical response to octreotide-LAR in acromegaly. J Clin Endocrinol Metab. 2009;94(6):1931-1937. 6. De Bruin C, Pereira AM, Feelders RA, et al. Coexpression of dopamine and somatostatin receptor subtypes in corticotroph adenomas. J Clin Endocrinol Metab. 2009;94(4):1118-1124. 7. Silverstein JM. Hyperglycemia induced by pasireotide in patients with Cushing’s disease or acromegaly. Pituitary. 2016;19(5):536-543. 8. Zambre Y, Ling Z, Chen MC, et al. Inhibition of human pancreatic islet insulin release by receptor-selective somatostatin analogs directed to somatostatin receptor subtype 5. Biochem Pharmacol. 1999;57(10):1159-1164. 9. Singh V, Brendel MD, Zacharias S, et al. Characterization of somatostatin receptor subtype-specific regulation of insulin and glucagon secretion: an in vitro study on isolated human pancreatic islets. J Clin Endocrinol Metab. 2007;92(2):673-680. 10. Breitschaft A, Hu K, Hermosillo Reséndiz K, Darstein C, Golor G. Management of hyperglycemia associated with pasireotide (SOM230): healthy volunteer study. Diabetes Res Clin Pract. 2014;103(3):458-465. 11. Henry RR, Ciaraldi TP, Armstrong D, Burke P, Ligueros-Saylan M, Mudaliar S. Hyperglycemia associated with pasireotide: results from a mechanistic study in healthy volunteers. J Clin Endocrinol Metab. 2013;98(8):3446-3453. 12. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540. 13. Lacroix A, Gu F, Gallardo W, et al. Efficacy and safety of once-monthly pasireotide in Cushing’s disease: a 12 month clinical trial. Lancet Diabetes Endocrinol. 2018;6(1):17-26. 14. Lacroix A, Gu F, Gallardo W, et al. Efficacy and safety of once-monthly pasireotide in Cushing’s disease: a 12 month clinical trial [supplemental material]. Lancet Diabetes Endocrinol. 2018;6(1):17-26.





